Abstract
Introduction
Myeloma (MM) is characterized by osteolytic bone disease, detected in up to 80% of patients, increasing the risk for skeletal related events (SREs), causing morbidity, undermining quality of life and impairing survival. Current imaging modalities provide qualitative measures of bone involvement, often after irreversible damage has occurred.
Bone strains are a biomechanical measure of bone strength which quantifies bone relative deformation in response to applied force. As a result of load on bone strains develop which may lead to fracture, once strain magnitude reaches the yield strain. Lytic bone lesions cause local weakening expressed by an increase in local strains.
We developed CT-based finite element analysis (CTFEA), a novel measure designed to accurately estimate bone strains based on conventional CT scans. We hypothesize this tool may enable quantifying changes in bone strength over time, by monitoring strain. In this concept study we explore the sensitivity and responsiveness of CTFEA in myeloma patients to detect bone-disease progression and treatment response, and explore clinical correlates.
Methods
Patients: A pilot retrospective study. After approval by the institutional ethics committee, 16 MM patients, with at least 2 consecutive CT scans including the spine and at least the proximal 1/3 of the femur since 2010 were identified. Demographic, clinical and radiographic data were abstracted from medical records.
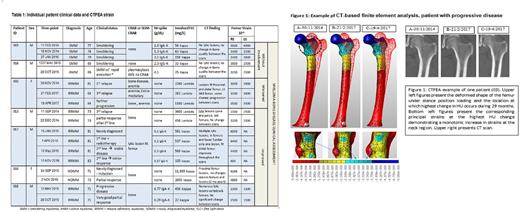
CTFEA: Femurs of interest were segmented from the CT scan, and a computational model was created with "stiffness" changing from point to point according to CT's gray values. The computational model was loaded virtually with a physiological stance position contact hip force (2.5 X body weight) applied on the femur's head. An elastic finite element analysis was performed that predicts the displacements and strains at any point within the bone (Figure 1). The locations of interest where the larger change in material properties may occur between consecutive CT scans were determined by computing the relative difference in Hounsfield unit (HU) radio-density along bone's axis. At these locations the principal strains were extracted and monitored. A monotonic increase in strains implies deterioration of bone strength, and vice versa. For patients with lytic lesions, bone strength is quantified by monitoring the principal strains in lesions vicinity. Healing is quantified by a decrease in strains. The CTFEA results were compared to conventional radiographic and laboratory measures.
Results
Table 1 summarizes individual patient clinical data at the time points of each of the CT-scans as well as the calculated CTFEA strain values at the location of maximum HU change of the initial 7 patients. Complete analysis of femur and vertebral strain for 16 patients will be presented at the meeting. Indeed no visible change in strain is demonstrated for smoldering MM (SMM) patients (Pts 15 and 16), whereas a monotonic increase is seen for patient 3, RRMM, with successive relapses without visible tumor identified in the femur in CT scans.
Patient 17 (RRMM) with a lytic tumor at the distal femur, shows an initial deterioration of bone strength followed by a monotonic improvement until almost full regain of strength after 19 months.
Patient 10 (NDMM) with a lytic tumor in the vicinity of the lesser trochanter, achieved a partial response of MM following anti-myeloma induction within 6 weeks, and as well as a significant increase in bone strength. Patient 13 had a rapid MM response but parallel worsening strain in the right femur, patient 18 had a MM partial response over 5 months with slight worsening of strain.
Conclusions
Promising trends were found between CTFEA strain prediction and MM clinical course. SMM patients had minimal changes whereas a patient with progressive disease had a monotonic increase in strains. A decrease in strain was seen in some but not all who responded to therapy. The preliminary study is to be enhanced by considering vertebrae, analyzing further patients as well as prospective validation against SREs, to refine calibrate and tailor this measurement. By providing a quantitative metric of bone strain, this tool may allow monitoring bone strength throughout myeloma evolvement, prior to development of SREs. This may support physicians making personalized medical and surgical treatment decisions and provide a useful surrogate endpoint for clinical drug development.
Yosibash: PerSimiO: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Trabelsi: PerSimiO: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal